Pediatric acute myeloid leukemia (AML) is a cancer with a particularly low mutational burden in comparison to other pediatric and adult cancers and therefore, thought to be a poor candidate for T cell-engaging immunotherapies (Gröbner et al., 2018; Pfister et al., 2022). However, little is known about the composition and function of T cells in the bone marrow (BM) of pediatric AML patients. Insight into the frequency and function of BM T cells in children with AML is relevant for naturally occurring AML immune defenses, as well as for T cell-engaging immunotherapies (Koedijk et al., 2021). Here, we performed a multidimensional characterization of the tumor immune microenvironment in children with newly diagnosed AML.
We obtained 82 formalin-fixed and paraffin-embedded (FFPE) diagnostic bone biopsies from a representative cohort of pediatric patients with treatment-naïve de novo AML (N=72) and from age- and sex-matched pediatric patients with treatment-naïve early-stage rhabdomyosarcoma without malignant BM infiltration (non-leukemic controls, N=10). We employed immunohistochemistry (IHC) alongside immune-related gene expression profiling (NanoString PanCancer IO 360 TM Panel) and spatial transcriptomics (NanoString GeoMx TM Whole Transcriptome Atlas). Moreover, we acquired an additional dataset of pre-treatment immune-related gene expression profiling obtained from the BM of 30 flotetuzumab-treated (CD3 x CD123 bispecific antibody) refractory/relapsed (R/R) adult AML patients (NCT02152956; Vadakekolathu et al., 2020) for TIDE-based immune deconvolution (Jiang et al., 2018).
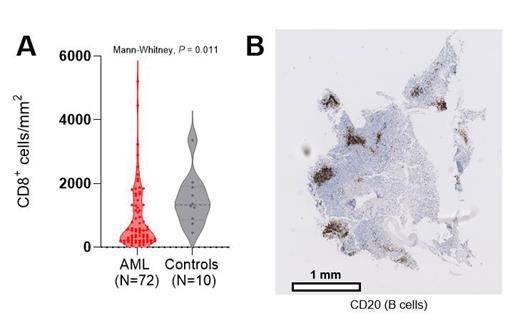
Using IHC, we identified (a trend towards) a decreased abundance of the overall number of T cells ( P=0.09) and CD8 + T cells ( P=0.011; Panel A) in the BM of pediatric AML patients in comparison to non-leukemic controls, respectively. Notably, the extent of overall T cell and CD8 + T cell infiltration could differ up to 180-fold between individual AML patients. In general, this difference in T cell infiltration was not associated with the abundance of leukemic blasts or patient's cytogenetic alterations. Nevertheless, children with complex karyotype AML had higher numbers of BM T cells compared to children with normal karyotype AML ( P=0.03).Using differential gene expression analysis, we identified genes related to T cell-attracting chemokines, cytotoxicity, and immune checkpoints to be significantly upregulated in T cell-infiltrated- compared to T cell-depleted samples. Moreover, the ratio of anti- to pro-inflammatory macrophages (M2/M1-ratio) was significantly lower in T cell-infiltrated- compared to T cell-depleted pediatric AML samples ( P<0.001). Interestingly, this M2/M1-ratio was also significantly lower in R/R adults that responded to flotetuzumab immunotherapy in comparison to non-responders ( P<0.001). In fact, this M2/M1-ratio outperformed several other known predictors of response to flotetuzumab immunotherapy (AUROC: 0.852 (95% CI: 0.71-0.99), P=0.001; Jiang et al., 2018; Ayers et al., 2017). In addition, IHC revealed that 9 immune-infiltrated samples, including 6 KMT2A-rearranged AML samples, harbored large networks of T- and B cells (representative image of B cell-networks shown in panel B). Using spatial transcriptomics, we dissected the composition of these lymphoid aggregates and revealed localized anti-tumor immunity in the BM of AML. In comparison to tumor areas, these aggregates showed a higher abundance of activated cytotoxic T cells ( P<0.001), memory B cells ( P<0.001), and plasma cells ( P<0.001), suggesting the presence of tertiary lymphoid structure-like (TLS-like) aggregates in the BM of AML.
In conclusion, we identified a subset of pediatric AML patients with relatively high T cell infiltration and a relatively low abundance of anti-inflammatory macrophages in the BM at diagnosis. Furthermore, we found that the BM M2/M1-ratio may be informative of response to T-cell engaging immunotherapies in adult AML, which needs to be validated in pediatric AML. Lastly, for the first time, we identified TLS-like aggregates in the BM of AML patients, which have been associated with immunotherapy response in many cancers (Schumacher & Thommen, 2022). Additional studies to further characterize the function and relevance of these lymphoid aggregates are ongoing.
Disclosures
Zwaan:Kura: Other: Institutional support for clinical trials; Gilead: Other: Institutional support for clinical trials; Novartis: Membership on an entity's Board of Directors or advisory committees; Syndax: Consultancy; Incyte: Consultancy; BMS: Consultancy; Kura Oncology: Consultancy; Jazz: Other: Institutional support for clinical trials; Gilead: Consultancy; Novartis: Consultancy; Daiichi Sankyo: Other: Institutional support for clinical trials; ITCC Hem Malignancies Committee: Other: Leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid; Chair Dutch MREC Society: Other: Leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid; Chair MREC Utrecht: Other: Leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid; Sanofi: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Takeda: Other: Institutional support for clinical trials; Abbvie: Other: Institutional support for clinical trials; Pfizer: Other: Institutional support for clinical trials. Heidenreich:Roche: Research Funding; Syndax: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal